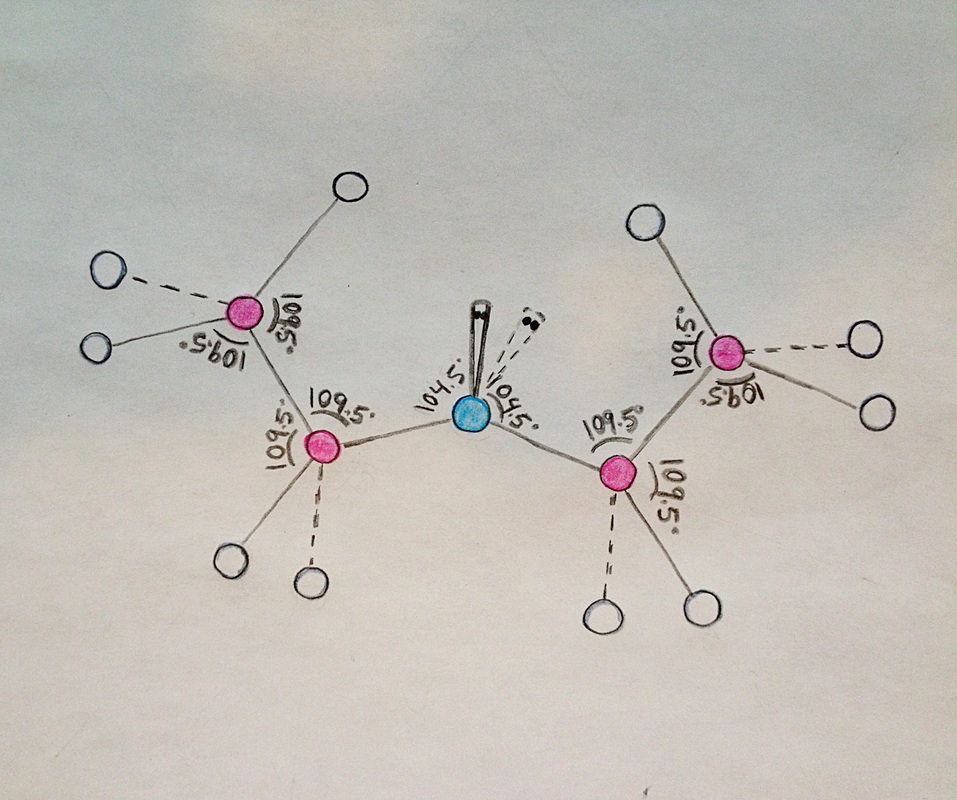
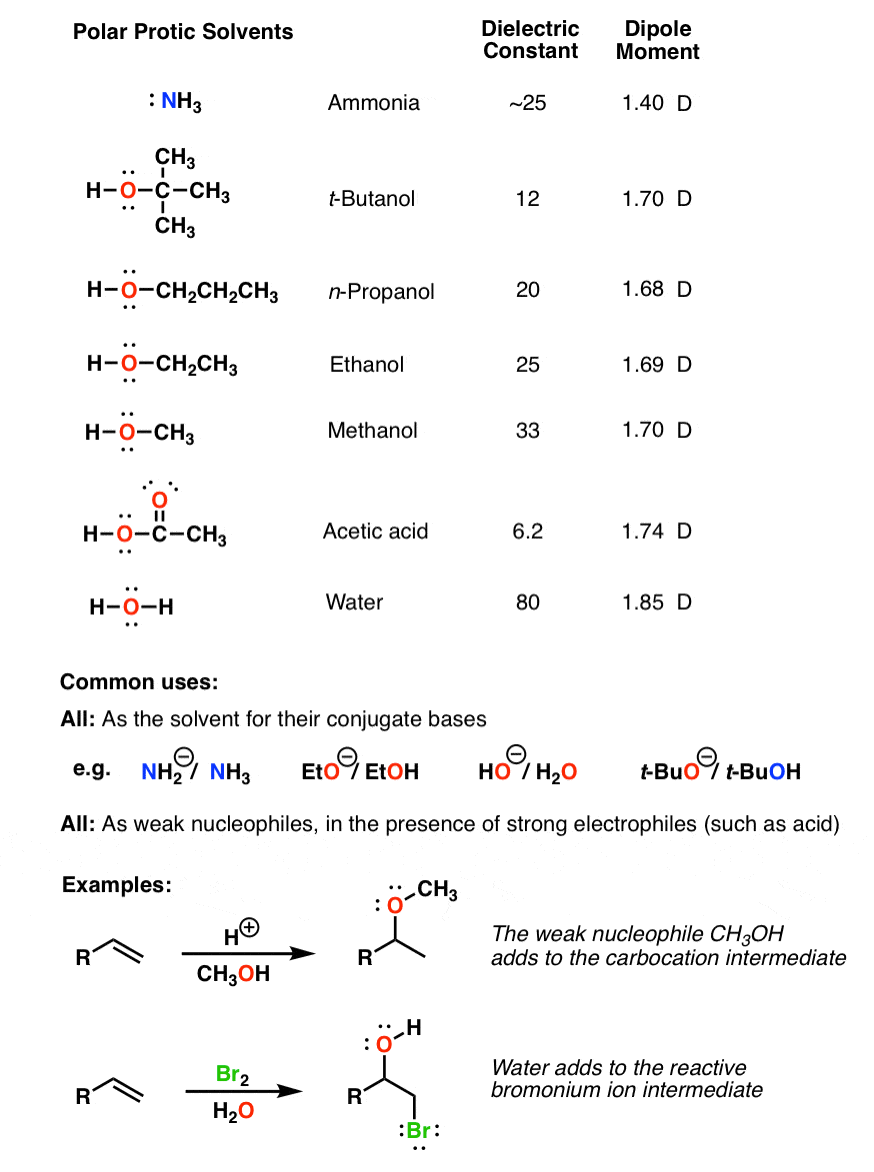
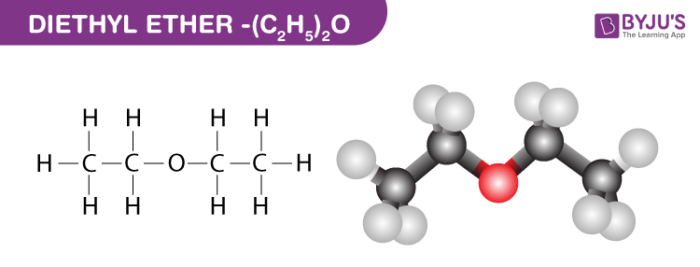
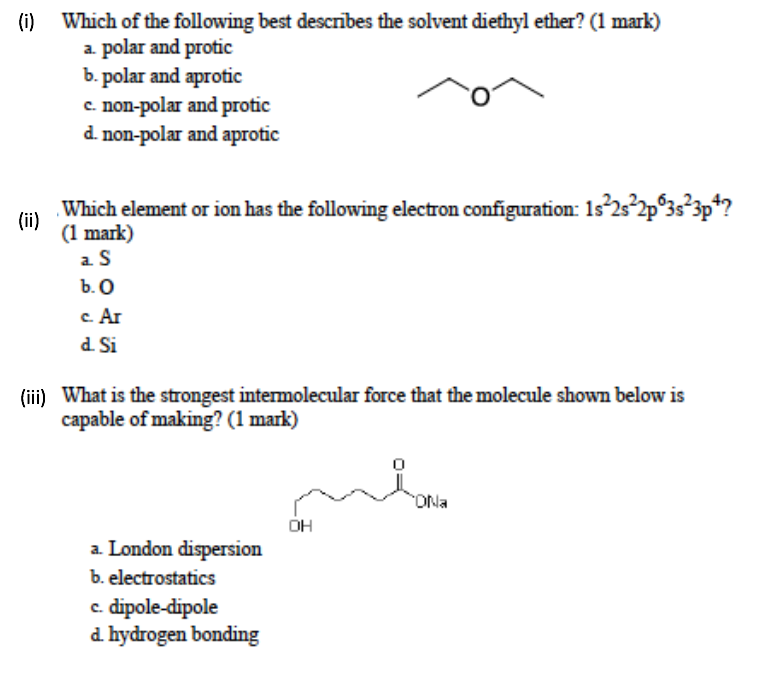
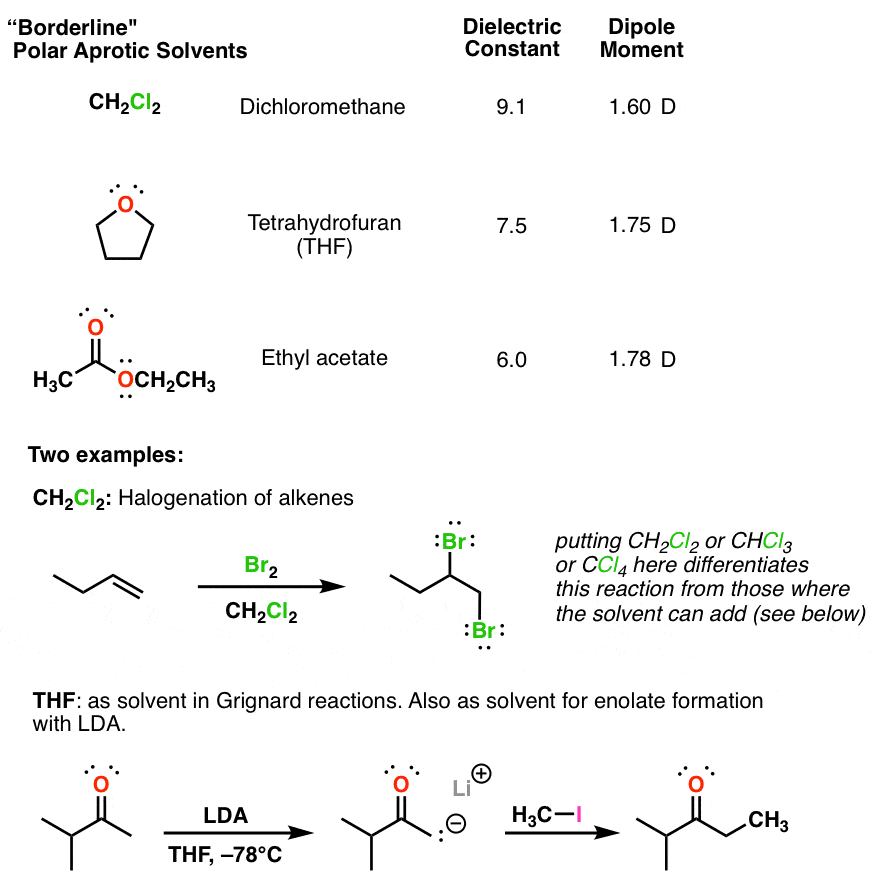
Diethyl ether has a much higher boiling point than butane despite having a higher molecular weight. Explain why this is the case, making reference to the molecular structures of both compounds.

State, whether the following statements are True or False: Ethers are more polar than the isomeric alcohols.